Allegheny Health Network’s media relations team is dedicated to providing reporters and other members of the news media with the assistance they need.
AHN Cardiovascular Institute at Allegheny General Hospital First in Region to Participate in International Clinical Trial for New, Minimally-Invasive Heart Failure Treatment
PITTSBURGH – Allegheny General Hospital (AGH), the flagship academic medical center of Allegheny Health Network (AHN), today announced it is the first hospital in western Pennsylvania to begin enrolling patients in a new clinical trial to evaluate a first-of-its-kind treatment option for heart failure.
Manreet Kanwar, MD, cardiologist and director of AHN’s heart failure division at the AHN Cardiovascular Institute along with AHN interventional cardiologists David Lasorda, DO and Mithun Chakravarthy, MD will lead a multidisciplinary team and work alongside heart centers around the world to study the safety and efficacy of a new device: the AccuCinch® Ventricular Restoration System.
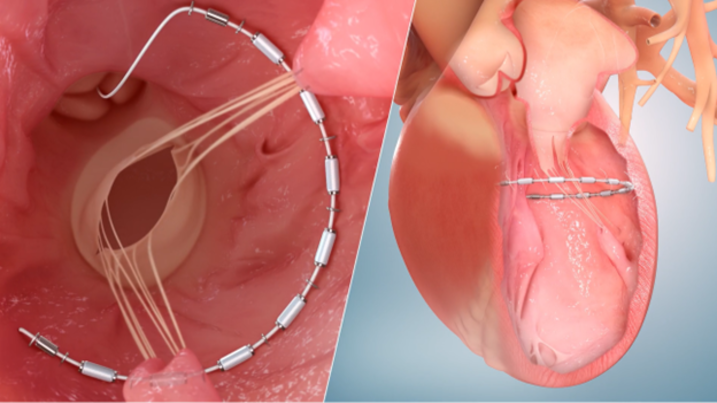
AGH interventional cardiologists implanted their first AccuCinch device in July as part of the global clinical trial, formally named the CORCINCH-HF study. The device aims to reduce the size of the heart’s left ventricle, improving the heart’s strength and blood-pumping function and mitigating heart failure symptoms.
“The AccuCinch device is an exciting advancement in the management of progressive heart failure, as it’s designed to improve heart function with a minimally invasive approach,” said Dr. Kanwar. “It’s our hope that its efficacy will result in our patients living fuller lives, with reduced cardiovascular symptoms and even a possible increase in life expectancy.”
Roughly 5.7 million Americans are living with heart failure, and the number continues to grow across the country and here in western Pennsylvania as the population ages.
Heart failure patients suffer from debilitating symptoms including persistent exhaustion, trouble breathing, swelling, confusion and loss of memory.
About half of all heart failure patients have an enlarged left ventricle, which is often caused by the heart overworking to adequately pump blood throughout the body. The left ventricle is the main pumping chamber, and when it becomes enlarged it causes more stress on the heart, leading to a reduced “ejection fraction” (the amount of blood that your heart pumps each time it beats).
During the AccuCinch procedure, a flexible implant is attached to the interior of the left ventricular wall and then cinched. The implant is intended to reduce the size of the left ventricle, reduce ventricular wall stress, and support and strengthen the heart wall, which in turn aims to minimize debilitating, progressive symptoms caused by heart failure.
“During this procedure, interventional cardiologists thread a catheter through the patient’s leg and track it into the left ventricle of the heart,” explained Dr. Kanwar. “When the cable is cinched, it acts like the string of a drawstring bag. Since it is a minimally invasive approach, we anticipate patients will be discharged the following day, getting them back to their daily routine quickly.”
AccuCinch is a viable option when existing therapies like lifestyle changes, medications and pacemakers are no longer able to manage the symptoms of heart failure. Early clinical data suggests the AccuCinch System may benefit patients who have heart failure with reduced ejection fraction, providing a new option that improves heart structure and function, and general quality of life.
“As a recognized leader in the treatment and management of progressive heart failure, AGH remains committed to participating in innovative clinical trials and research initiatives that help to shape the future of cardiovascular medicine,” said Stephen Bailey, MD, cardiothoracic surgeon and Chair of AHN Cardiovascular Institute. “Today’s announcement is a testament to our longstanding legacy of transforming cardiovascular care not only for patients throughout the region, but around the world.”
The CORCINCH-HF Study includes roughly 80 heart centers from around the world, including AGH. The study will enroll 400 patients and is being conducted to support a Premarket Approval (PMA) application to the U.S. Food and Drug Administration (FDA). Enrolled patients will be randomized to receive treatment with the AccuCinch System and guideline-directed medical therapy, or guideline-directed medical therapy alone.
For more information about this clinical study, and to find out if you might be a candidate for enrollment, please contact 412-359-6181.
Link to Download Animation:
https://www.dropbox.com/s/guy0lw0hokkpm2t/Ancora%20UPDATED%20Restoration%20Animation%208_21_20%20%281080p%29.mp4?dl=0
###
About The CORCINCH-HF Study and the AccuCinch System
Enrolled patients will be randomized to receive treatment with the AccuCinch System plus guideline-direction medical therapy or guideline-directed medical therapy alone. To be eligible, patients must meet the following main criteria:
- Have been told they have heart failure by their doctor
- Have had their doctor explain that they have reduced ejection fraction (low heart pumping ability)
- Are taking heart failure medications, but have symptoms that are worsening (e.g., shortness of breath, fatigue, coughing, leg swelling, or trouble breathing at night)
The AccuCinch System is an investigational device, which is currently being studied in the CORCINCH-HF pivotal trial, a prospective, randomized, open-label, multicenter, international, clinical safety and efficacy investigation (ClinicalTrials.gov Identifier: NCT04331769). Early clinical data suggests that the system may provide an effective treatment option by filling the gap between medication or cardiac resynchronization therapy and left ventricular assist devices (LVADs) or a heart transplant. The AccuCinch System was developed by Santa Clara, California-based Ancora Heart. Additional information is available at www.ancoraheart.com.

