Allegheny Health Network’s media relations team is dedicated to providing reporters and other members of the news media with the assistance they need.
Pittsburgh’s Allegheny General Hospital to Participate in Early Impella® Support in Patients with ST-Segment Elevation Myocardial Infarction Complicated by Cardiogenic Shock: The RECOVER IV Internaptional Trial
PITTSBURGH -- Doctors at Allegheny General Hospital plan to study a small heart pump in patients with heart attacks who have a condition called cardiogenic shock. A heart attack, also called a myocardial infarction, happens when a part of the heart muscle does not get enough blood. Cardiogenic (heart) shock is when the heart is not pumping enough blood to the rest of your body.
Usually, a heart attack is treated by a percutaneous coronary intervention (PCI) in which a thin tube (catheter) is placed through a blood vessel in the patient’s arm or leg to open an artery that is blocked near the heart. In patients with heart shock, the additional use of a temporary heart pump can move blood to the rest of the body before, during and after the PCI. The best strategy to improve outcomes for patients with heart shock is unknown.
“We’re looking forward to contributing to this study at AGH, and learning if this treatment protocol improves survival rates for heart attack patients experiencing cardiogenic shock. If it does, this study could establish a new standard for heart attack care,” said Manreet Kanwar, MD, heart failure specialist at Allegheny General Hospital.
Some doctors already use a temporary heart pump when treating this kind of heart attack as part of their standard treatment, and some doctors do not.
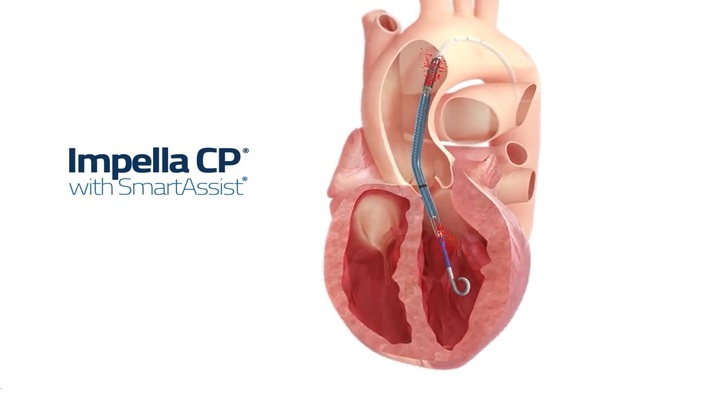
This study is being done to learn if it would be better for heart recovery to use a temporary heart pump to treat patients with heart attack and shock. The temporary heart pump is called the Impella CP®. This has been approved by the FDA for use in patients with cardiogenic shock, but we do not know whether it improves survival.
Eligible patients will be randomly assigned to receive standard heart attack care with or without the Impella CP heart pump. If they receive the Impella CP, it will be inserted into their leg artery through a thin tube (catheter) and advanced through the blood vessels into the main pumping chamber of their heart. It then helps move blood from this chamber to the rest of the body. It will stay in for at least 24 hours and will be removed when their doctor thinks it is appropriate. If they do not receive the Impella CP, doctors will treat them with all standard treatments).
All patients will receive standard care if they join this study. If they also receive the Impella CP, the device may help the pumping function of their sick heart, reduce the need for medications during care, reduce problems and improve survival and quality of life. There are known potential risks of the Impella CP heart pump. These include bleeding, stroke, infection, heart rhythm problems, damage to blood cells or heart tissues. There may be other risks.
This study is sponsored by Abiomed, Inc. of Danvers, MA. Doctors and researchers in over 50 hospitals across the United States and Europe plan to enroll at least 560 patients into RECOVER IV. The study is approved by the Food and Drug Administration (FDA) and an independent institutional review board (IRB). It will be monitored by an independent group of experts.
Heart shock is a life-threatening emergency and treatment decisions must be made very quickly. Most patients will be too sick due to their illness to give their informed consent to participate in the study within the initial emergency treatment window. In many cases, a family member or other representative may not be available to make decisions for them. A special set of research regulations allow people to be included in studies like this without consent under carefully controlled circumstances. This research helps improve emergency care medicine that could not otherwise happen without these exceptions.
Community members can learn more about the RECOVER IV Study here https://redcap.link/R4-agh They may opt out of potential future enrollment if they wish to do so at https://redcap.link/R4optout
###

