
Newsroom

AHN Physician Dr. Maria Gioia to Receive Prestigious Nathaniel Bedford Primary Care Award
Thursday, November 13, 2025
This esteemed award from the Allegheny County Medical Society recognizes a primary care physician for exemplary, compassionate, comprehensive and dedicated care of patients.
AHN in the News
-
KDKA-AM: Seasonal affective disorder
Dec 08, 2025
-
KDKA-AM: Chron's and Colitis
Dec 01, 2025
-
KDKA-AM: GERD Awareness Week
Nov 24, 2025
-

-

AHN Welcomes Allegheny Ophthalmic & Orbital Associates into AHN Ophthalmology
Thursday, December 11, 2025
Allegheny Health Network today announced the acquisition of Allegheny Ophthalmic & Orbital Associates, an independent eye care and eye surgery practice based at AHN Allegheny General Hospital.
-

AHN Cancer Institute Unveils 2026 Saturday Cancer Screening Schedule
Wednesday, December 10, 2025
Registration is now open for the Saturday, Jan. 10 screening at AHN West Penn Hospital
-

AHN Neuroscience Institute to Launch Dedicated Neuroscience Track within AlphaLab Health
Tuesday, December 09, 2025
Aimed at solving complex neurological challenges, the new neuroscience track in partnership with AlphaLab Health is made possible through generous grant funding
-
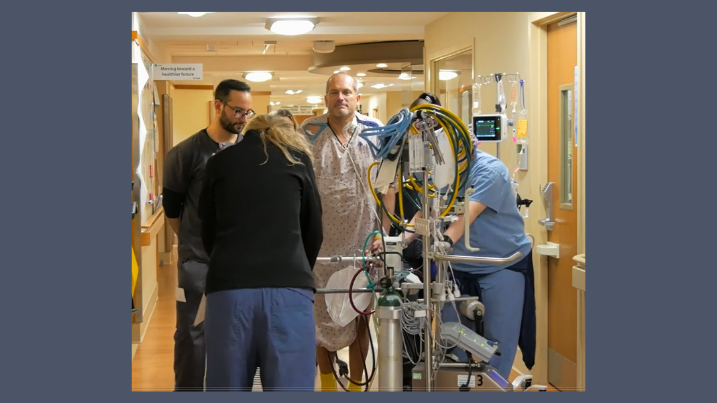
AHN Allegheny General Hospital reports strong health outcomes for patients who can stand, walk while receiving heart-lung bypass therapy
Wednesday, December 03, 2025
A new program at AHN Allegheny General Hospital demonstrates that getting patients up and moving around while receiving ECMO support can dramatically improve health outcomes, leading to better survival rates and shorter hospital stays.
-

AHN’s Monroeville Surgery Center Ranked #1 in Pennsylvania in Newsweek List of America’s Best Ambulatory Surgery Centers
Tuesday, December 02, 2025
Three other AHN Facilities Lauded on State’s Top 10 List

Building the future
Allegheny Health Network and Highmark Health are investing more than $1 billion over the next decade in new construction and hospital expansions, to advance community access to affordable, high-quality health care in western Pennsylvania.

AHN Apps and Podcasts
AHN podcasts share insights from health care pros and personal journeys from AHN patients. AHN apps can transform the way you receive health care, allowing you to review your medical information and connect with a doctor on your schedule.
Are you a media professional?
Allegheny Health Network’s media relations team is dedicated to providing reporters and other members of the news media with the assistance they need.
By accessing this video, I understand that I am leaving the AHN website and I will be re-directed to an external website operated by a third party platform provider. I acknowledge that the platform provider may collect personal information about me, and about the video that I view, on their platform and may use and disclose this information in accordance with its privacy policy. I agree that Allegheny Health Network is not responsible for the data collection and use practices of this third party.

