Leading the Region in Cardiac Care: AHN Performs First TTVR Procedure in Western Pennsylvania
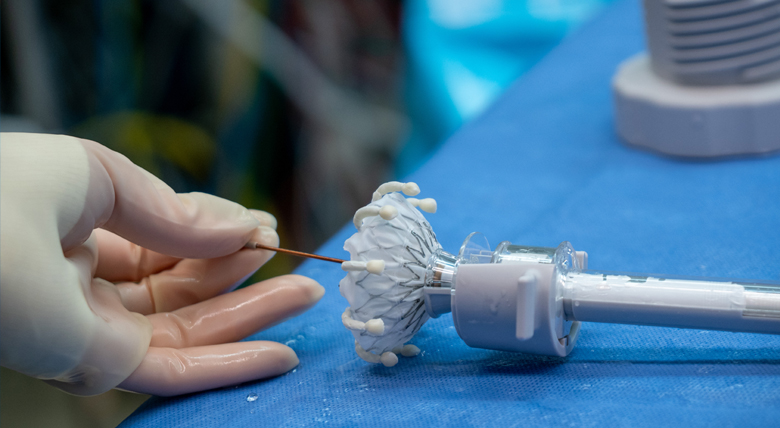
Surgeons at AHN Cardiovascular Institute have completed the region’s first transcatheter tricuspid valve replacement (TTVR) to treat tricuspid valve regurgitation using the EVOQUE system, a newly FDA-approved replacement technology from Edwards Lifesciences.
The minimally invasive procedure, performed at AHN Allegheny General Hospital (AGH) by Cardiac Surgeon, Walter McGregor, MD, Interventional Cardiologist, David Lasorda, MD, and Interventional Echocardiologist, Georgios Lygouris, MD, marks a critical expansion of structural heart capabilities now available to referring clinicians across western Pennsylvania.
Addressing a critical need
Tricuspid valve regurgitation affects an estimated 1.6 million people in the U.S. and results from incomplete closure of the tricuspid valve, most commonly from congenital defects, infection, or trauma.* The resulting backflow of blood into the right atrium places added demand on the heart and can ultimately lead to end-organ dysfunction.
Symptoms generally manifest only in advanced stages of disease and may include:
- Extreme fatigue
- Exertional dyspnea
- Jugular venous pulsations or palpitations
- Peripheral or abdominal edema
Before TTVR, management was largely limited to diuretics and afterload reduction, with high surgical risk often precluding open tricuspid valve surgery. This gap has created a substantial unmet need for patients who become symptomatic but are not candidates for conventional intervention.
Providing a new option with TTVR
TTVR offers a minimally invasive, near immediate solution for symptoms and long-term disease management.
The EVOQUE valve — consisting of a self-expanding nitinol frame, a sealing skirt, and bovine pericardial tissue leaflets — is available in multiple sizes to treat varying disease severity and patients’ unique physiologic anatomy.
During TTVR, a catheter is inserted through the femoral vein and advanced to the tricuspid valve. The prosthetic EVOQUE valve is then deployed, expanding in situ to restore valvular function.
The procedure delivers clinical benefits including:
- Shorter hospitalization and recovery periods
- Rapid symptomatic improvement
- Enhanced response to guideline-directed medical therapy
“TTVR is a groundbreaking option for patients with symptomatic tricuspid valve regurgitation. Within two weeks, patients report relief of symptoms and a return to their lives,” said Dr. McGregor. “Being the first to provide this to patients in our region underscores AHN’s commitment to advancing innovative therapies that create real, immediate change.”
Identifying candidates for TTVR
Early referral enables comprehensive evaluation and clinical decision-making, both essential to optimal outcomes. Consider TTVR evaluation for patients with:
- Severe symptomatic tricuspid valve regurgitation (primary or secondary).
- Persistent symptoms despite optimized medical therapy.
- Mild-to-moderate right heart ventricular dysfunction or dilation, but not end-stage right heart failure.
- Functional limitations affecting daily activity or exercise tolerance.
Comprehensive evaluation at AHN includes transthoracic echocardiography, transesophageal echocardiography (TEE), and occasionally, cardiac CT to assess the severity of tricuspid valve regurgitation, the valve’s anatomy, and the right ventricular size and function.
AHN’s multidisciplinary structural heart team reviews each case collaboratively, integrating imaging, clinical status, comorbidities, and patient goals to determine appropriateness for TTVR or alternative therapies.
How to refer
AHN Cardiovascular Institute’s team of renowned cardiologists, surgeons, and heart specialists are highly experienced in all major valvular diseases and structural heart conditions. To refer a patient for TTVR evaluation or to consult with our team, call 844-MD-REFER 844-637-3337 or submit a referral online.
About Walter McGregor, MD
Walter E. McGregor, MD is an accomplished cardiac surgeon who offers expertise in mitral valve disease, robotic heart surgery, transcatheter mitral repair, and complex surgical reconstructive techniques.
As the Director of Allegheny Health Network's Cardiac Surgery Division, he has developed a multidisciplinary mitral disease program offering unique treatments to patients in Western Pennsylvania that incorporate robotic and catheter-based technologies for enhanced recovery and improved outcomes.
At AHN Cardiovascular Institute, our renowned cardiologists, surgeons, and heart specialists are committed to keeping your heart beating longer and stronger. Our specialists focus on providing expert care for conditions like heart attack and heart failure, as well as preventive medicine to keep you healthy.

